Abstract
Introduction
Autologous stem cell transplant (ASCT) remains the standard therapy for newly diagnosed multiple myeloma (NDMM) patients who are eligible, with improved clinical outcomes. Within the Asia-Pacific (APAC) region, there is only limited information on real world treatment data and ASCT utilisation for MM. ASCT has been reported to have increased by 148% over the last decade, however, there are likely to be disparities in the utilization of ASCT within the different APAC nations, either due to lack of expertise or financial resources. This study aims to determine the demographic pattern and treatment outcome of MM patients proceeding with ASCT in 3 APAC jurisdictions - Malaysia, a middle-income nation and 2 other developed nations, Korea, and Singapore. These data may be useful in providing an understanding of the relationship between transplant activities and socioeconomic factors.
Methods
This was a retrospective analysis of NDMM patients who proceeded to ASCT in Malaysia, Singapore, and Korea. The inclusion period was from September 2018 to June 2021. Patient information from Singapore and Korea was obtained from APAC Myeloma and Related Disease Registry (MRDR) whereas for Malaysian patients' data were obtained from the medical records from 3 major hospitals. Patient demographics, disease characteristics, therapies prior to ASCT, disease status pre- and post-ASCT and clinical outcome (overall survival [OS] and progression-free survival [PFS]) were analysed. Patients were followed up for a minimum of 2 years from diagnosis.
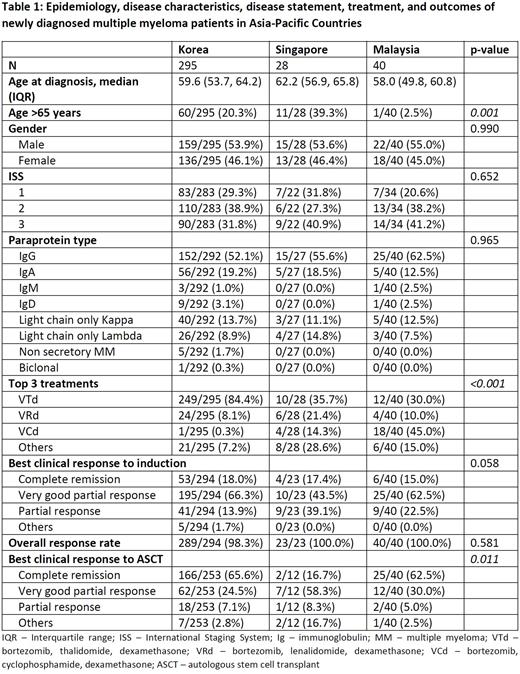
Results
A total of 363 NDMM patients who underwent ASCT were included. Patients with MM who had ASCT were comparatively younger in Malaysia (median age 58.0 years), compared to Korea (59.6 years) and Singapore (62.2 years). The majority were males in all 3 nations. The International Staging System (ISS) was available for 339 patients with the majority being Stage 2 in Korea (38.9%) but Stage 3 in both Malaysia (41.2%) and Singapore (40.9%). Immunoglobulin G (IgG) was the most common paraprotein encountered in all countries. There was significant difference in the induction regimens used between the 3 nations. Bortezomib, thalidomide and dexamethasone (VTd) was most common in Korea (84.4%) and Singapore (37.5%), whereas bortezomib, cyclophosphamide and dexamethasone (VCd) was predominantly used in Malaysia (45.0%) (p<0.001). Clinical response to induction treatment was similar in all participating countries, where majority of patients achieved very good partial response (VGPR) (p=0.058). Post-ASCT, most Korean and Malaysian patients (65.6% and 62.5%, respectively) achieved complete remission (CR), while Singaporeans (58.3%) were predominantly classified as VGPR (p=0.011). Median PFS for patients who underwent ASCT in Korea was not reached compared to patients in Singapore, 26.9 months, and 21.0 months for Malaysian patients. However, there was no difference in OS.
Conclusion
Although there is disparity in epidemiology and clinical practice among NDMM patients in various APAC jurisdictions, this study shows that ASCT is widely utilised and has an ongoing role in the era of novel agent as a recommended part of consolidation therapy for eligible NDMM patients.
Disclosures
Tan:Amgen: Honoraria; BMS: Honoraria; Novartis: Honoraria, Research Funding; MSD: Honoraria. Syed Abd Kadir:MSD: Research Funding. Aoki:Janssen-Cilag: Research Funding. Wood:Novartis: Research Funding; Janssen-Cilag: Research Funding; Gilead: Research Funding; CSL Behring: Research Funding; Beigene: Research Funding; Bristol-Myers Squibb: Research Funding; Astra Zeneca: Research Funding; Antengene: Research Funding; Amgen: Research Funding; Abbvie: Research Funding; Sanofi: Research Funding; Roche: Research Funding. Spencer:Amgen: Consultancy, Honoraria; Haemalogix: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria. Chng:Hummingbird: Research Funding; Takeda: Honoraria; Novartis: Honoraria; Abbvie: Honoraria; BMS: Honoraria; Celgene: Honoraria, Research Funding; J&J: Honoraria, Research Funding; Amgen: Honoraria. Gan:Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal